Atsumaru Holdings Inc. is now offering high-quality silk for medical applications to be sold worldwide, and it is collaborating with VAXESS Technologies, a U.S. biotech startup


Silk protein is expected to be a next-generation bioabsorbable material, but due to concerns about the production environment, traceability, and supply stability, its use in products that are expected to be marketed has not materialized for a long time.
In order to overcome this problem, Atsumaru has established a consistent quality control system unique to the purpose-built plant. This includes pesticide-free cultivation of mulberry leaves in the mulberry garden managed by Atsumaru Yamaga Silk Inc. complete cocoon production in a self-contained facility, established production procedure, and documented traceability from raw materials to final product.
Starting with the collaboration with VAXESS, Atsumaru will maximize the potential of high-quality silk and work to solve social issues in the medical field.
Advantages of high-quality silk in medical device applications
1. An unparalleled level of cleanliness from breeding all ages in a self-contained clean environment
2. Traceability by completing all processes in-house, from mowing up (hatching) to cocoon harvesting and silk reeling
3. Quality control based on ISO9001 certification
4. Completely pesticide-free cultivation of mulberry leaves in the Dedicated mulberry farm
Overview for VAXESS company and product


The slow-release, bioactive tips that make this possible are made from silk fibroin proteins—unlike any other existing technology.
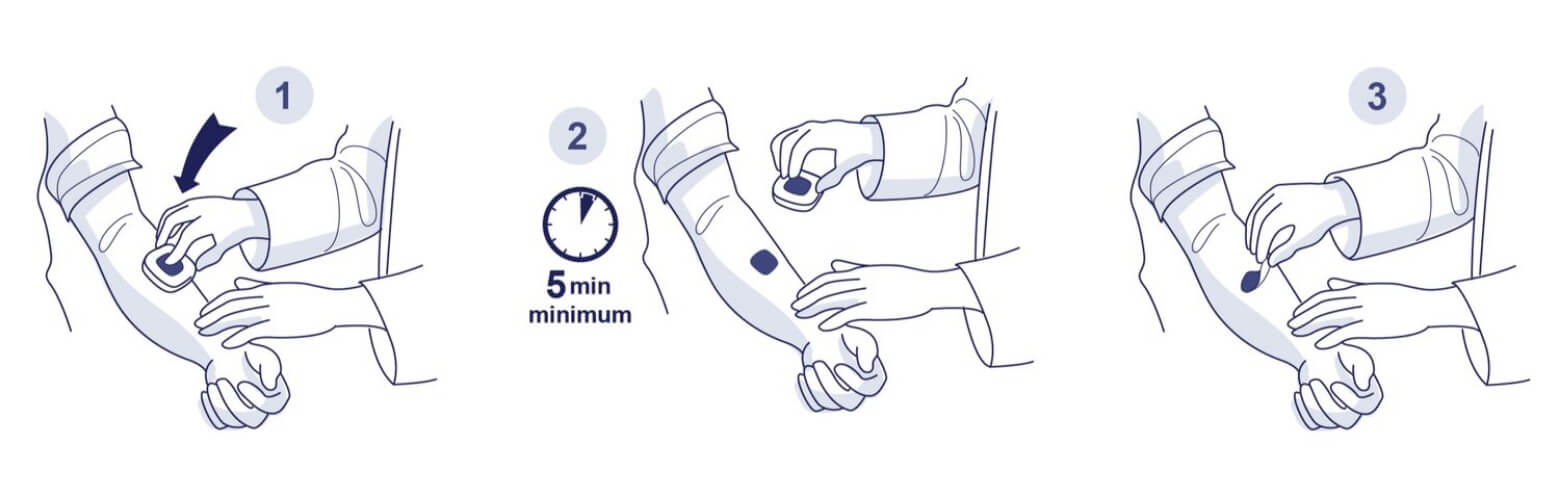
Assumed future schedule


